Calcium chloride (or
Calcium dichloride,
Calcium(II) chloride).
Chemical formula : CaCl2 (anhydrous)
(Cl = 63,89 %, Ca = 36,11 %, )
Molar mass = 110,984 ± 0,008 g·mol-1
The anhydrous salt is a very hygroscopic white powder.
It is deliquescent; it can accumulate enough water in its crystal
lattice to form a solution.
Calcium chloride crystallizes
from water to form three different hydrates:
- monohydrate
(CaCl2.H2O), 128.999
g/mol
- dihydrate
(CaCl2.2H2O), 147.014
g/mol
- tetrahydrate
(CaCl2.4
H2O), 183.045 g/mol
- hexahydrate (CaCl2.6
H2O), 219.08 g/mol
Production.
It is mainly obtained from chalk (calcium carbonate
formed).
For example it may be prepared by reacting calcium carbonate
[CaCO3] and hydrochloric solution [HCl] acid:
CaCO3 + 2 HCl >>> CaCl2 +
H2O + CO2
Calcium chloride can be produced directly from limestone, but
large amounts are also produced as a by product of the Solvay
process.
This is a very hygroscopic material should be stored in tightly
closed containers. It is prized for its exothermic qualities.
It behaves like a typical halide salt, in particular with a good
electrical conductivity in the liquid state and ionic chemical
bonds.
In water treatment, it is used by injection in the form of water
solutions (saturated or not).
The most widely used commercial solutions are:
- 33% or 17.7 g / L (density 1.33 - crystallization temperature
- 30 ° C)
- 42% or 34.1 g / L (density 1.41 - crystallization temperature
+ 18 ° C of )
Main Features :
|
|
Anhydrous form
|
|
% CaCl2
|
>96 %
|
|
Volumetric mass density
(20°C)
|
2150 kg/m3
|
|
Melting point
|
772°C
|
|
Solubility at 20°C
|
745 g/L
|
Solubility of anhydrous calcium chloride in water (soft)
depending on the temperature (CaCl2 g / kg of H2O):
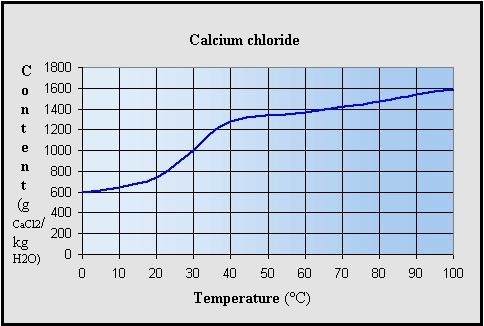
The dissolution process is exothermic and above 60 °C can be
reached rapidly.
Due to its very hygroscopic nature, it can be used to dry the air,
gas or other body fluids (or seeds).
When it absorbs water or water vapor of the substance to be dried, it
is transformed into brine:
CaCl2 + 2 H2O >>>
CaCl2.2H2O
A formulation for water
treatment.
Injection of 11.1 mg of CaCl2 in 1 liter of pure water,
calcium hardness (calcium) increases by 4 mg/L of calcium (or
1°F).
Standardization.
Standard products used for the production of drinking water:
French
Official Bulletin - Calcium chloride
Uses.
- Food:
- As an ingredient, it is listed as a permitted food additive
in the European Union for use as a sequestrant and firming
agent with the E number E509. It is considered as generally
recognized as safe (GRAS) by the U.S. Food and Drug
Administration and is on the US National Organic Program's
National List of Allowed and Prohibited Substances.The average
intake of calcium chloride as food additives has been estimated
to be 160–345 mg/day for individuals.
- As a firming agent, calcium chloride is used in canned
vegetables, in firming soybean curds into tofu and in producing
a caviar substitute from vegetable or fruit juices. It is
commonly used as an electrolyte in sports drinks and other
beverages, including bottled water. The extremely salty taste
of calcium chloride is used to flavor pickles while not
increasing the food's sodium content. Calcium chloride's
freezing-point depression properties are used to slow the
freezing of the caramel in caramel-filled chocolate bars.
- In brewing beer, calcium chloride is sometimes used to
correct mineral deficiencies in the brewing water. It affects
flavor and chemical reactions during the brewing process, and
can also affect yeast function during fermentation. Calcium
chloride is sometimes added to processed milk to restore the
natural balance between calcium and protein in casein for the
purposes of making cheeses, such as brie, Pélardon and
Stilton. Also, it is frequently added to sliced apples to
maintain texture.
- Other :
- Calcium chloride is used in concrete mixes to help speed up
the initial setting, but chloride ions lead to corrosion of
steel rebar, so it should not be used in reinforced
concrete.The anhydrous form of calcium chloride may also be
used for this purpose and can provide a measure of the moisture
in concrete.
- Calcium chloride is used in swimming pool water as a pH
buffer.
- Calcium chloride is included as an additive in plastics and
in fire extinguishers, in wastewater treatment as a drainage
aid, in blast furnaces as an additive to control scaffolding
(clumping and adhesion of materials that prevent the furnace
charge from descending), and in fabric softener as a
thinner.
- The exothermic dissolution of calcium chloride is used in
self-heating cans and heating pads.
- In the oil industry, calcium chloride is used to increase
the density of solids-free brines. It is also used to provide
inhibition of swelling clays in the water phase of invert
emulsion drilling fluids.
- CaCl2 acts as flux material (decreasing melting point) in
the Davy process for the industrial production of Sodium metal,
through the electrolysis of molten NaCl.
- Calcium chloride is also an ingredient used in ceramic
slipware. It suspends clay particles so that they float within
the solution making it easier to use in a variety of
slipcasting techniques.
Safety.
Calcium chloride is irritating. It should be handled with gloves. It
is relatively safe to handle, but it should not be ingested, it
reacts exothermically with water, it can cause burning of the mouth
or esophagus.
Sources : personal and Wikipedia,
the free encyclopedia.
 (use your browser)
(use your browser)


 (use your browser)
(use your browser)