Calcium sulfate (or
calcium sulphate)
Chemical formula : CaSO4 (anhydrous form)
(Ca = 29.5 %, S = 23.5 %, O = 47 % )
Molar mass (M) = 136,14 g·mol-1
In the natural state, unrefined calcium sulfate is a translucent,
crystalline white rock.
Notes: heating the gypsum between 100 °C and 150 °C.,
partially dehydrates the mineral by driving about 75% of water
contained in its chemical structure. The temperature and time
required depends on the partial pressure of water. Temperatures of
170 °C are used in industrial calcination, but at these
temperatures the anhydrite is starting to form.
The reaction of partial dehydration is:
The partially dehydrated mineral is called calcium sulfate
hemihydrate or calcined gypsum ("plaster of
Paris") (CaSO4·nH2O), where n is in the range 0.5 to
0.8.
In contrast to most minerals, which when rehydrated simply form
liquid or semi-liquid pastes, or remain powdery, calcined gypsum has
an unusual property: when mixed with water at normal (ambient)
temperatures, it quickly reverts chemically to the preferred
dihydrate form, while physically "setting" to form a rigid and
relatively strong gypsum crystal lattice:
This reaction is exothermic and is responsible for the ease with
which gypsum can be cast into various shapes including sheets
(for drywall), sticks (for
blackboard chalk), and molds (to immobilize
broken bones, or for metal casting). Mixed with polymers, it
has been used as a bone repair cement. Small amounts of calcined
gypsum are added to earth to create strong structures directly from
cast earth, an alternative to adobe (which loses its
strength when wet). The conditions of dehydration can be
changed to adjust the porosity of the hemihydrate, resulting in the
so-called alpha and beta hemihydrates (which are more
or less chemically identical).
On heating to 180 °C, the nearly water-free form, called y-anhydrite (CaSO4·nH2O where n = 0 to 0.05) is produced. y-Anhydrite reacts slowly with water to return to the dihydrate state, a property exploited in some commercial desiccants. On heating above 250 °C, the completely anhydrous form called ß-anhydrite or "natural" anhydrite is formed.
Natural anhydrite does not react with water, even over geological
timescales, unless very finely ground.
Commercial production and recovery.
The main sources of calcium sulfate are naturally occurring
gypsum and anhydrite which occur at many locations worldwide as
evaporites. These may be extracted by open-cast quarrying or by deep
mining. World production of natural gypsum is around 127 million
tonnes per annum.[
In addition to natural sources, calcium sulfate is produced as a
by-product in a number of processes:
These precipitation processes tend to concentrate radioactive
elements in the calcium sulfate product. This is particularly the
case with the phosphate by-product, since phosphate rocks naturally
contain actinides.
Main properties :
|
Product > |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

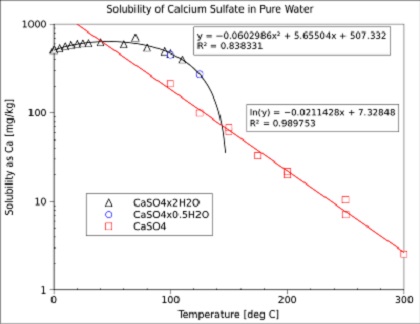
Calcium sulfate is an important component of fouling deposits in
industrial heat exchangers. This comes from its solubility decreases
with increasing temperature (see above Figure).
Note that the solubility of calcium sulfate in these steps is
approximately 10 times higher than which is calculated with the
solubility product (?).
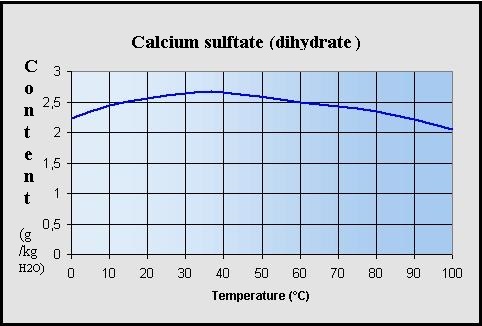
Maximum solubility of calcium sulfate dihydrate
[CaSO4.2H2O]
in soft water (g / kg H2O)
:
Uses.
In the form of y-anhydrite (the nearly anhydrous
form), it is used as a desiccant. It is also used as a
coagulant in products like tofu. In the natural state, unrefined
calcium sulfate is a translucent, crystalline white rock.
When sold as a color-indicating variant under the name Drierite, it
appears blue or pink due to impregnation with Cobalt(II) chloride,
which functions as a moisture indicator.
The hemihydrate (CaSO4·~0.5H2O) is better
known as plaster of Paris, while the dihydrate (CaSO4·2H2O)
occurs naturally as gypsum.
The anhydrous form occurs naturally as ß-anhydrite. Depending
on the method of calcination of calcium sulfate dihydrate, specific
hemihydrates are sometimes distinguished: alpha-hemihydrate and
beta-hemihydrate. They appear to differ only in crystal shape.
Alpha-hemihydrate crystals are more prismatic than beta-hemihydrate
crystals and, when mixed with water, form a much stronger and harder
superstructure.
In the treatment of water, it is mainly used for injection in the
form of water saturated solutions 1 to 2 g / L.
A formulation for water treatment.
Injection of 13,6 mg of CaSO4 in 1 liter of pure
water, calcium hardness (calcium) increases by 4 mg/L as Ca (or
1°F).
Storage.
Store in a cool, dry, well ventilated area away from heat and
moisture, and incompatible products.
Safety.
This product does not present a risk to health by inhalation,
ingestion or contact unless subjected to operations that can generate
the formation of particles in the air, and exposure to large amounts
of dust may cause irritation of skin, eyes, nose, throat, or upper
respiratory tract.
If in eyes, do not rub or scratch. To prevent mechanical irritation,
flush thoroughly with water for 15 minutes. If irritation persists,
consult a doctor.
Above 1450 ° C, decomposes to calcium oxide (CaO, or
"quicklime") and sulfur dioxide (SO2).
See also > International
Chemical Satefy Card 1215 (Canada) or
NIOSH
Pocket Guide to Chemical Hazards (USA)
Sources : personal and Wikipedia,
the free encyclopedia.
 (use your browser)
(use your browser)