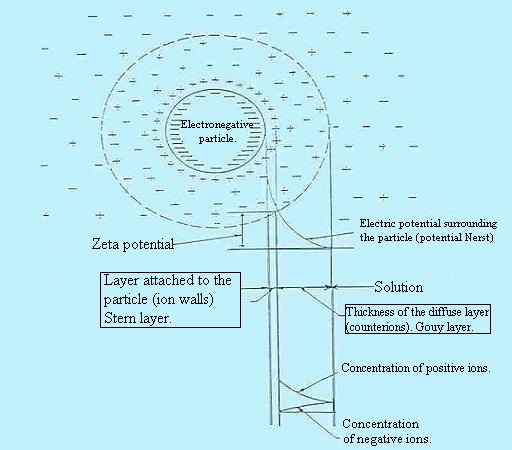
Principles.
Ion distribution around a colloidal
particle
(Gouy-Chapman Theory ) :
Louis Georges Gouy in 1910 and David Leonard Chapman in 1913 both
observed that capacitance was not a constant and that it depended on
the applied potential and the ionic concentration. The "Gouy-Chapman
model" made significant improvements by introducing a diffuse model
of the DL. In this model the charge distribution of ions as a
function of distance from the metal surface allows
Maxwell–Boltzmann statistics to be applied. Thus the electric
potential decreases exponentially away from the surface of the fluid
bulk.
Gouy-Chapman model fails for highly charged DLs. In 1924 Otto Stern
suggested combining Helmholtz with Gouy-Chapman. In Stern's model,
some ions adhere to the electrode as suggested by Helmholtz, giving
an internal Stern layer, while some form a Gouy-Chapman diffuse
layer. The Stern layer accounted for ions' finite size and
consequently ions' closest approach to the electrode is on the order
of the ionic radius. The Stern model had its own limitations,
effectively modeling ions as point charges, assuming all significant
interactions in the diffuse layer are Coulombic, assuming dielectric
permittivity to be constant throughout the double layer and that
fluid viscosity is constant above the slipping plane.

with,
. Center: colloidal ion;
. Outside: free ions and electric charge equivalent to that of the
opposite ion and colloidal sign forming two layers:
Reminders about the nature of the colloidal particles and
the stability of colloidal dispersions.
The vast majority of natural waters (surface water especially ),
contains impurities that affect their appearance and may have adverse
effects for the consumer. These solids can be roughly classified as
follows:
Actually, this covers the classification of particle size
intuitively relevant grasped that is the most difficult to remove the
finer particles. To this notion of dimension, there are two other
more important are those of the surface / volume of colloidal
particles which gives them very pronounced adsorption properties and
that of their surface charge report.
This charge may come from:
For their study, colloids are classified arbitrarily into two main groups;
More generally, these are mineral products.
The existence of colloidal systems depends on the interaction between
two particles. It involves two opposing forces:
Destabilization of colloids.
To overcome the repulsive forces, two possibilities can be
theoretically considered:
Electrostatic stabilization and steric stabilization are the two main mechanisms for stabilization against aggregation.
A combination of the two mechanisms is also possible
(electrosteric stabilization). All the above
mentioned mechanisms for minimizing particle aggregation rely on the
enhancement of the repulsive interaction forces.
Under these conditions, the particles are close enough to each other
so that the van der Waals forces become predominant.
The process leading to this result is usually called
coagulation..
Destabilization (flocculation).
Unstable colloidal dispersions can form flocs as the particles
aggregate due to interparticle attractions. In this way photonic
glasses can be grown. This can be accomplished by a number of
different methods:
Unstable colloidal suspensions of low-volume fraction form
clustered liquid suspensions, wherein individual clusters of
particles fall to the bottom of the suspension (or float to the top
if the particles are less dense than the suspending medium) once the
clusters are of sufficient size for the Brownian forces that work to
keep the particles in suspension to be overcome by gravitational
forces. However, colloidal suspensions of higher-volume fraction form
colloidal gels with viscoelastic properties. Viscoelastic colloidal
gels, such as bentonite and toothpaste, flow like liquids under
shear, but maintain their shape when shear is removed. It is for this
reason that toothpaste can be squeezed from a toothpaste tube, but
stays on the toothbrush after it is applied.
The coagulating nature of reagents.
Theoretically, any type of electrolyte can be used for coagulation /
flocculation of colloid. However, it is recognized, for many years,
that the efficiency of a coagulant increases dramatically with the
oxidation number (valence) of the cation of the electrolyte.
This is why the aluminum salts and ferric iron containing cations of
oxidation number +3 are used almost exclusively in the coagulation /
flocculation of water.
The salts of aluminum and iron present in addition to their higher
valency, are of interest to hydrolysis in the usual pH range of
natural waters, giving an insoluble hydroxide precipitates.
We know, however, recently, that is not the trivalent ion that plays
an essential role, but the intermediate hydrolysis products
formed.
Aluminum salts of iron and lead in effect, in dilute solution, the
following hydroxo-complexes:
(1) X+++ + OH- << >> X (OH)++(2) X (OH)++ + OH- << >> X (OH)+2
(3) X (OH)+2 + OH- << >> X(OH)3
(4) X(OH)3 + OH- << >> X(OH)-4
where OH = hydroxyl ions, X = Al or Fe
Although equilibrium, these reactions are substantially complete
in the usual pH of the waters, and irreversible at least for the
first three.
These hydrolysis products have a very strong tendency to polymerize
to give the first (polyhydroxo complexes) and soluble macromolecular
colloidal buildings, and insoluble hydroxides finally.
The relative proportion of each of these complexes depend on the
environmental conditions (pH, temperature,
concentration).
It has been shown that it may be preferable to use an aluminum salt
partially neutralized with sodium hydroxide under conditions to
promote the formation of a particular complex before injection into
the water.
This technique is among others responsible for the marketing of WAC
(Water Aluminium Chloride) and PCBA (Polyvinyl Chloride Basic
Aluminum).
Influençants flocculation parameters:
There is a pH optimum flocculation for water type and a given
flocculant: this pH result from the raw water, the action of the
coagulant which consumes OH- ions and the possible
introduction of a reactive controller.
The usual pH values in natural waters, not existing OH-
free ions, they result from the decomposition of bicarbonate
(HCO3-) by the flocculant
(and formation of carbon dioxide CO2):
reaction which is superimposed the balance of calcium bicarbonate with its dissociation products.
The overall type of coagulation equation can be written:
therefore, precipitation of a metal hydroxide X(OH)3 and CO2 formation.
Recall CO2 + H2O <<< >>> H2CO3 (carbonic acid).
The appearance of carbonic acid and the subsequent decrease in bicarbonate alkalinity leads to a lowering of the pH of the medium whose magnitude depends on the buffering capacity (initial alkalinity and ionic strength), and the introduced coagulant dose. For this reason, it is essential to consider the pH, alkalinity and mineralization of raw water for the process definition coagulation / flocculation.
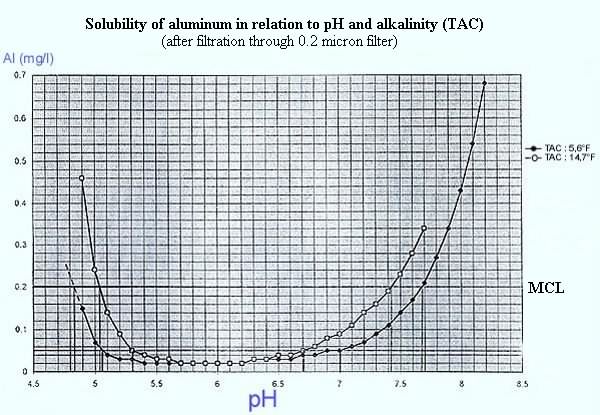
It may be necessary to obtain a correct flocculation, to adjust
the pH of the water by adding either an acid or a base
(sodium hydroxide, lime, sodium
carbonate).
Other general factors involved in the coagulation-flocculation, are
following:
On the contrary, the inorganic colloids are easily flocculated by low doses of coagulant unrelated intensity turbidity.
Main reagents coagulants.
(click on the name for more information)
Aluminum sulphate
(or alum) :
Aluminum hydroxides are formed within a fairly narrow pH range,
typically: 5.5 to about 7.7 (Optimum 6)
Empirical formula: Al2(SO4)3, n
H2O
(commercial product n = 14, n = 18 per pure product)
It is available in various solid forms (depending on the
manufacturer):
Ferric
chloride (or
iron(III)
chloride) :
To form over a larger pH range including pH levels lower than are
effective for alum, typically: 5.0 to 8.5 (Optimum 8).
Formula: FeCl3 (pure product).
For the treatment of water, it is only used as an aqueous solution of
about 592 g as FeCl3 /L
(41 wt%).
It is stored in tanks, in containers or tanks.
Chlorosulfate ferric:
Its empirical formula is FeClSO4.
For the treatment of water, it is only used in the form of aqueous
solution of about 200 g as FeClSO4 /L.
(about 41% by weight).
It is stored in tanks, containers or tanks : PVC materials,
polyethylene, polypropylene, acid resistant rubber, glass or
ceramic.
WAC
(PAC
or
polyaluminium chloride)
:
This is a basic aluminum polychloride with an empirical formula: Aln
(OH) m-Cl3n m (wherein m/3n is comprised between 0.45 and 0.60).
It is in the form of a liquid with a content of A1203 is about
10%.
The pH range for use is from 6 to 7.5 (optimum 6.5).
It is stored in tanks.
other:
Also include sodium aluminate, alone or in conjunction with alum or
ferric chloride, ferric sulfate or ferrous sulfate.
>>links to French official "bulletin" : Circulaire
DG 5/VS 4 n° 2000-166 of 28 March 2000, in relation to the
products of treatment processes (water intended for
human consumption)
Choice of reagents and determination of treatment rates.
• Choice of coagulant:
A number of parameters must be considered:
• Purposefulness of treatment rates:
Coagulation and flocculation are complex phenomena influenced by many
parameters:
also the safest and most effective method to determine in each
case the nature and quantity of reagent to be used, will be based on
experimentation.
The method which reproduces the small-scale overall process of
flocculation-coagulation is the so-called JAR TEST, used in
laboratory.
In this research the best possible result (which must
also take into account economic considerations), the
experience of man shall be assisted by the flocculation test
laboratory (JAR-TEST), and possibly by implementation of pilot
test.
 (use your browser)
(use your browser)