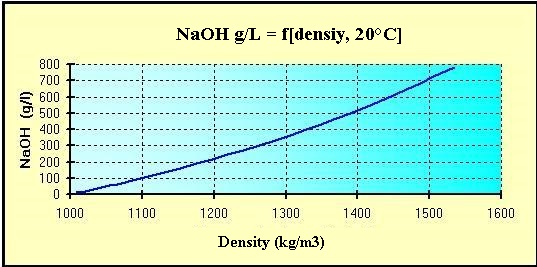
The maximum solubility in fresh water (20 °C) is 1 090 g / L.
Sodium hydroxide
(caustic soda, lye).
Chemical formula : NaOH
(Na = 57,48 %, O = 40 %, H = 2,52 %)
Molar mass = 39,9971 ± 0,0004 g·mol-1
Production.
Historically, sodium hydroxide was produced by treating sodium
carbonate with calcium hydroxide in a metathesis reaction. (Sodium
hydroxide is soluble while calcium carbonate is not.) This process
was called causticizing:
This process was superseded by the Solvay
process in the late 19th century, which was in turn supplanted by
the chloralkali process which we use today.
Sodium hydroxide is also produced by combining pure sodium metal with
water. The byproducts are hydrogen gas and heat, often resulting in a
flame, making this a common demonstration of the reactivity of alkali
metals in academic environments; however, it is not commercially
viable, as the isolation of sodium metal is typically performed by
reduction or electrolysis of sodium compounds including sodium
hydroxide.
Sodium hydroxide is industrially produced as a 50% solution by
variations of the electrolytic chloralkali process. Chlorine gas is
also produced in this process. Solid sodium hydroxide is obtained
from this solution by the evaporation of water.
Process being replaced (2020) by electrolysis membrane.
Also from sodium carbonate (caustication): this technique was that
previously used. It is still used in North America where natural
deposits of sodium carbonate. This is an addition of lime (Ca
[OH] 2) with sodium carbonate [Na2CO3]. The reaction
is written:
It is a white solid and highly caustic metallic base and alkali salt
which is available in pellets, flakes, granules, and as prepared
solutions at a number of different concentrations.
The dissolution in water of sodium hydroxide is known as lye.
Similar to the hydration of sulfuric acid, dissolution of solid
sodium hydroxide in water is a highly exothermic reaction in which a
large amount of heat is liberated, posing a threat to safety through
the possibility of splashing. The resulting solution is usually
colourless and odorless with slippery feeling upon contact in common
with other alkalis.
In treatment of water, injection is used in water diluted solid
product (with softened water) or commercial solutions (30 and 50%
NaOH by weight).
Main Features :
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

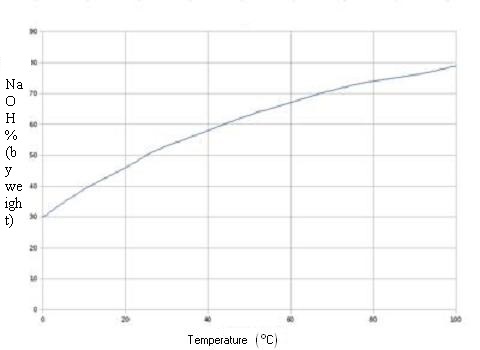
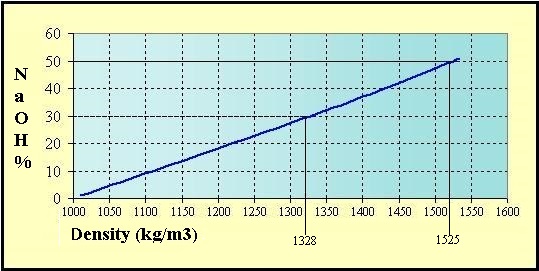
Quantity of pure sodium hydroxide by the Volumetric mass
density [d] of the solutions at 20 °C:
(d = kg/m3 ou g/L)
A formulation for water
treatment.
Reaction mechanism of lime with aggressive CO2 (simplified
equations):
CO2 + NaOH >>> NaHCO3
.44
.........40
...........84 or 50 mg/L as CaCO3
(5°F<TAC)
To neutralize 1 mg of CO2, it is necessary to use
(40/44)= 0.91 mg as NaOH, which
form(84/44)=1.91 mg of sodium bicarbonate NaHCO3, and
therefore also the neutralization of 1 mg /L to form CO2 (5/44) =
0.1136 °F/l de TAC (i.e.1.136 mg CaCO3/L of Alkalinity).
Note: if the calculated dose is a reagent for 100% purity, it is
necessary to correct this dose to entering the purity of the
commercial product.
Standardization.
Standard products used for the production of drinking water:
French
Official Bulletin - Sodium hydroxide, Caustic soda : NF EN
896.
Effects on the environment.
Caustic soda increases the pH of the stream, thus representing a
potential threat to aquatic fauna and flora (stream, river,
groundwater).
Uses.
Sodium hydroxide is a popular strong base used in the industry.
Around 56% of sodium hydroxide produced is used by the industry, 25%
of which is used in paper industry. Sodium hydroxide is also used in
manufacturing of sodium salts and detergents, pH regulation, and
organic synthesis.
It is used in the Bayer process of aluminium production. In bulk it
is most often handled as an aqueous solution, since solutions are
cheaper and easier to handle.
Other:
Safety.
Chemical burns caused by sodium hydroxide solution photographed
44 hours after exposure.
Like other corrosive acids and alkalis, drops of sodium hydroxide
solutions can decompose proteins and lipids in skin, eyes or other
living tissues via amide hydrolysis and ester hydrolysis, which
consequently causes chemical burns and may induce permanent blindness
if it contacts eyes. Solid alkali may also express its corrosive
nature if there is water, so protective equipment such as rubber
gloves, safety clothing and eye protection should always be used when
handling the material or its solutions.
Moreover, dissolution of sodium hydroxide is highly exothermic, and
the resulting heat may cause heat burns or ignite flammables. It also
produces heat when reacted with acids.
The standard first aid measures for alkali spills on the skin is, as
for other corrosives, irrigation with large quantities of water.
Washing is continued for at least ten to fifteen minutes.
Sodium hydroxide is also mildly corrosive to glass, which can cause
damage to glazing or freezing of ground glass joints. Careful storage
is needed.
Sources : personal and Wikipedia,
the free encyclopedia.
 (use browser)
(use browser)