GENERALITY :
Chlorine (gas phase) is the result of combining two chlorine Cl
atoms, or the formula Cl2.
Molecular weight: 70.906 g mol-1.
Chemical compound of the family of halogens, chlorine is a
yellowish-green gas with suffocating smell very unpleasant and it is
extremely toxic.
Chlorine has the highest electron affinity and the fourth highest
electronegativity of all the reactive elements. For this reason,
chlorine is a strong oxidizing agent. Free chlorine is rare on Earth,
and is usually a result of direct or indirect oxidation by
oxygen.
The high oxidizing potential of elemental chlorine led commercially
to free chlorine's bleaching and disinfectant uses, as well as its
many uses of an essential reagent in the chemical industry. Chlorine
is used in the manufacture of a wide range of consumer products,
about two-thirds of them organic chemicals such as polyvinyl
chloride, as well as many intermediates for production of plastics
and other end products which do not contain the element. As a common
disinfectant, elemental chlorine and chlorine-generating compounds
are used more directly in swimming pools to keep them clean and
sanitary.
Properties.
non-flammable gas (but supports combustion in the
air).
It is a heavier-than-air gas = 3.2 kg
m3-1 (0 °C,
101.325 kPa).
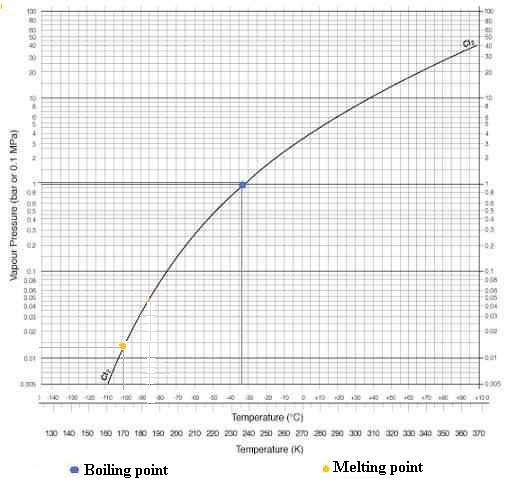
Boiling point = -34.04 °C (239.11 K).
Liquid density at b.p. = 1.5625 g·cm3.
Melting point = -101.5 °C (171.6 K).
Critical point = 416.9 K, 7.991 MPa.
Triple Point is located at T = - 101.00 ° C (172 K) and P = 14
hPa (0.014 bar)
Heat of fusion (Cl2) = 6.406
kJ·mol-1.
Heat of vaporization (Cl2) = 20.41
kJ·mol-1.
Chlorine gas is normally carried in the liquid state, in
pressurized steel cylinders.
------------------------------
Chlorine phase diagram:
(Sources : Air Liquide)
Some features::
- Gas phase:
- Density (1013.25 hPa, at 15 °C
) : 3.04 kg m3-1
- Equivalent gas / liquid (1013.25 hPa
15 °C) : 1 m3 as gas (3.04 kg) >
0.001919 m3 as liquid
- Specific volume (1013.25 hPa, 21 °C
) : 0.336 m3 kg-1
- Specific heat at constant pressure (Cp)
(1013.25 hPa at 25
°C) : 33 J/(mole.K)
- Viscosity (1013.25 hPa, at 0 °C
) : 0.0001245 Poise
- Thermal Conductivity (1013.25 hPa, at 0
°C ) : 7.91 mW/(m.K)
- Solubility in water (1013.25 hPa, at 0
°C ) : 4.61 vol/vol.
Note: 10 ° C, 1 liter of water dissolved 3.10 liters of
chlorine, and 1.77 liter at 30 ° C.
- Liquid phase:
- Density (1013.25 hPa,
at boiling point) : 1562.5 kg
m3-1
- Equivalent gas / liquid (1013.25
hPa at 15 °C) : 521 vol/vol
( 1 m3 as liquid chlorine > 521 m3 as gas
[1583.839 kg] )
- Boiling point (1013.25 hPa) : -34.1
°C (239.05 K)
- Latent heat of vaporization (1013.25 hPa,
at boiling point) : 287.79 kJ kg-1
- Vapor pressure (21 °C) : 0.695
MPa (6.95 bar).
- Solid phase (ice):
- Melting point : -100.84°C (172.31
K)
- Latent heat of fusion (1013.25 hPa,
triple point ) : 90.374 kJ
kg-1
---------------------------
Standardization.
Standard products used for the production of drinking water :
bulletin
officiel - Chlore : NF EN 937.
Uses.
- Production of industrial and consumer products.
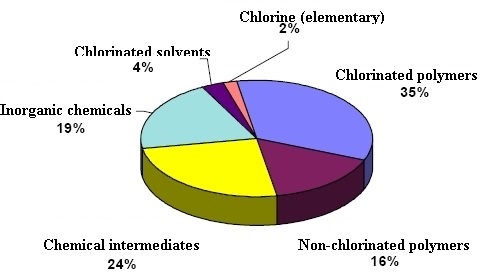
Principal applications of chlorine are in the production of a
wide range of industrial and consumer products. For example, it is
used in making plastics, solvents for dry cleaning and metal
degreasing, textiles, agrochemicals and pharmaceuticals,
insecticides, dyestuffs, household cleaning products, etc.
Many important industrial products are produced via organochlorine
intermediates. Examples include polycarbonates, polyurethanes,
silicones, polytetrafluoroethylene, carboxymethyl cellulose, and
propylene oxide. Like the other halogens, chlorine participates in
free-radical substitution reactions with hydrogen-containing
organic compounds. When applied to organic substrates, reaction is
often—but not invariably—non-regioselective, and, hence,
may result in a mixture of isomeric products. It is often
difficult to control the degree of substitution as well, so
multiple substitutions are common. If the different reaction
products are easily separated, e.g., by distillation, substitutive
free-radical chlorination (in some cases accompanied by concurrent
thermal dehydrochlorination) may be a useful synthetic route.
Industrial examples of this are the production of methyl chloride,
methylene chloride, chloroform, and carbon tetrachloride from
methane, allyl chloride from propylene, and trichloroethylene, and
tetrachloroethylene from 1,2-dichloroethane.
Quantitatively, about 63% and 18% of all elemental chlorine
produced is used in the manufacture of organic and inorganic
chlorine compounds, respectively,. About 15,000 chlorine compounds
are being used commercially.The remaining 19% is used for bleaches
and disinfection products. The most significant of organic
compounds in terms of production volume are 1,2-dichloroethane and
vinyl chloride, intermediates in the production of PVC. Other
particularly important organochlorines are methyl chloride,
methylene chloride, chloroform, vinylidene chloride,
trichloroethylene, perchloroethylene, allyl chloride,
epichlorohydrin, chlorobenzene, dichlorobenzenes, and
trichlorobenzenes. The major inorganic compounds include HCl,
Cl2O, HOCl, NaClO3, chlorinated isocyanurates, AlCl3, SiCl4,
SnCl4, PCl3, PCl5, POCl3, AsCl3, SbCl3, SbCl5, BiCl3, S2Cl2, SCl2,
SOCI2, ClF3, ICl, ICl3, TiCl3, TiCl4, MoCl5, FeCl3, ZnCl2, etc
Public sanitation, disinfection, and antisepsis.
Bleach (water chlorination).
Combating putrefaction.
In France (as elsewhere) there was a need to process animal
guts in order to make musical instrument strings, Goldbeater's
skin and other products. This was carried out in "gut factories"
(boyauderies) as an odiferous and unhealthy business. In or about
1820, the Société d'encouragement pour l'industrie
nationale offered a prize for the discovery of a method, chemical
or mechanical, that could be used to separate the peritoneal
membrane of animal intestines without causing putrefaction. It was
won by Antoine-Germain Labarraque, a 44 year-old French chemist
and pharmacist who had discovered that Berthollet's chlorinated
bleaching solutions ("Eau de Javel") not only destroyed the smell
of putrefaction of animal tissue decomposition, but also retarded
the decomposition process itself.
Labarraque's research resulted in chlorides and hypochlorites of
lime (calcium hypochlorite) and of
sodium (sodium hypochlorite) being employed
not only in the boyauderies but also for the routine disinfection
and deodorisation of latrines, sewers, markets, abattoirs,
anatomical theatres and morgues. They were also used, with
success, in hospitals, lazarets, prisons,
infirmaries (both on land and at sea),
magnaneries, stables, cattle-sheds, etc.; and for exhumations,
embalming, during outbreaks of epidemic illness, fever, blackleg
in cattle, etc.
Water treatment.
Chlorine is usually used (in the form of hypochlorous acid) to
kill bacteria and other microbes in drinking water supplies and
public swimming pools. In most private swimming pools, chlorine
itself is not used, but rather sodium hypochlorite, formed from
chlorine and sodium hydroxide, or solid tablets of chlorinated
isocyanurates. The drawback of using chlorine in swimming pools is
that the chlorine reacts with the proteins in human hair and
skin (Hypochlorous acid, see bleach).
Once the chlorine reacts with the hair and skin, it becomes
chemically bonded. Even small water supplies are now routinely
chlorinated.
It is often impractical to store and use poisonous chlorine gas
for water treatment, so alternative methods of adding chlorine are
used. These include hypochlorite solutions, which gradually
release chlorine into the water, and compounds like sodium
dichloro-s-triazinetrione (dihydrate or
anhydrous), sometimes referred to as "dichlor", and
trichloro-s-triazinetrione, sometimes referred to as "trichlor".
These compounds are stable while solid and may be used in
powdered, granular, or tablet form. When added in small amounts to
pool water or industrial water systems, the chlorine atoms
hydrolyze from the rest of the molecule forming hypochlorous acid
(HOCl), which acts as a general biocide, killing germs,
micro-organisms, algae, and so on.
Health effects of the free element and hazards :
- Chlorine is a toxic gas that irritates the respiratory system.
Because it is heavier than air, it tends to accumulate at the
bottom of poorly ventilated spaces. Chlorine gas is a strong
oxidizer, which may react with flammable materials.
Chlorine is detectable with measuring devices in concentrations of
as low as 0.2 parts per million (ppm), and by smell at 3 ppm.
Coughing and vomiting may occur at 30 ppm and lung damage at 60
ppm. About 1000 ppm can be fatal after a few deep breaths of the
gas. Breathing lower concentrations can aggravate the respiratory
system, and exposure to the gas can irritate the eyes. The
toxicity of chlorine comes from its oxidizing power. When chlorine
is inhaled at concentrations above 30 ppm, it begins to react with
water and cells, which change it into hydrochloric acid (HCl) and
hypochlorous acid (HClO).
When used at specified levels for water disinfection, the reaction
of chlorine with water is not a major concern for human health.
Other materials present in the water may generate disinfection
by-products that are associated with negative effects on human
health, however, the health risk is far lower than drinking
undisinfected water.
High exposure to chlorine can cause induced asthma : Reactive
Airways Dysfunction Syndrome or RADS is a term proposed by Stuart
M. Brooks M.D. and colleagues in 1985 to describe an asthma-like
syndrome developing after a single exposure to high levels of an
irritating vapor, fume, or smoke. It involves coughing, wheezing,
and dyspnea. It can also manifest in adults with exposure to high
levels of chlorine, ammonia, acetic acid or sulphur dioxide,
creating symptoms like asthma. The severity of these symptoms can
be mild to fatal, and can even create long-term airway damage
depending on the amount of exposure and the concentration of
chlorine. Some experts classify RADS as occupational asthma. Those
with exposure to highly irritating substances should receive
treatment to mitigate harmful effects.
Toxicity:
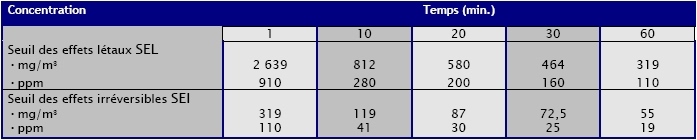
(Source : INERIS -
03DR002.doc/Septembre2003)
Link
(Canadian Centre for Occupational Health and
Safety).
> Sources (link)
: BELGOCHLOR (BelgoChlor, Diamant Building, Bd
A.Reyers 80, 1030 Bruxelles)
Download Technical MSDS AL (pdf files > over
there ).
 (use your browser)
(use your browser)




 (use your browser)
(use your browser)